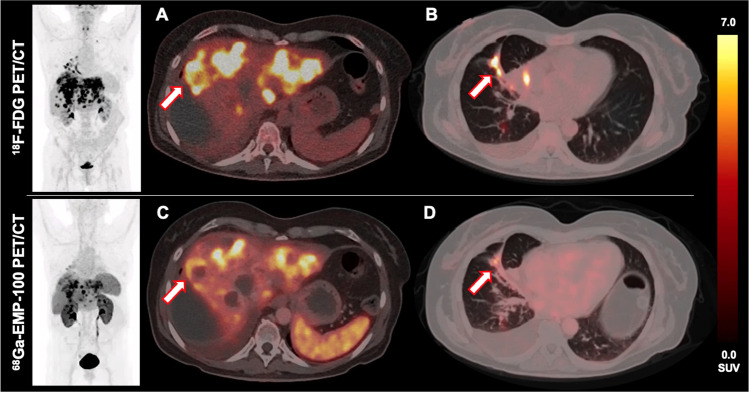
A case of a 56-year-old female former smoker with stage IV EGFR mutation-positive NSCLC (lymphogenic, pulmonary, hepatic, and cerebral metastases is presented. Stage: cT3, cN3, cM1b) and concomitant MET exon 14 skipping mutation. After multiple pretreatments including radiochemotherapy and additional systemic therapies with afatinib, crizotinib, osimertinib, and cabozantinib, the patient underwent 18F-FDG PET/CT for treatment monitoring purposes, where multiple pulmonary and hepatic metastases with high glucose consumption were noted (Fig. 1A, B). Moreover, the patient underwent 68Ga-EMP-100 PET/CT (6 days before 18F-FDG PET/CT) to further assess c-MET expression in these tumoral sites. Here, a highly heterogeneous c-MET expression was found with some lesions showing high c-MET expression, but also lesions with only slight or even barely any increased c-MET expression despite their high glucose consumption on 18F-FDG PET (see Fig. 1 A vs. C and Fig. 1 B vs. D). Overall, elevated tumor metabolism was not necessarily accompanied by high c-MET expression..
Conclusion: This case illustrates the heterogeneity of tumor molecular biology in the stage IV setting, and the potential of 68Ga-EMP-100 PET/CT for pre-therapeutic and non-invasive assessment of whole-body c-MET expression in NSCLC patients prior to c-MET targeting tyrosine kinase inhibitors such as cabozantinib. Tissue biopsies may underestimate the heterogeneity of tumor lesions. Molecular imaging may provide a broader assessment of the distribution and relevance of particular molecular features, such as the c-MET expression as assessed with 68Ga-EMP-100 PET/CT, and thus inform treatment priorities..