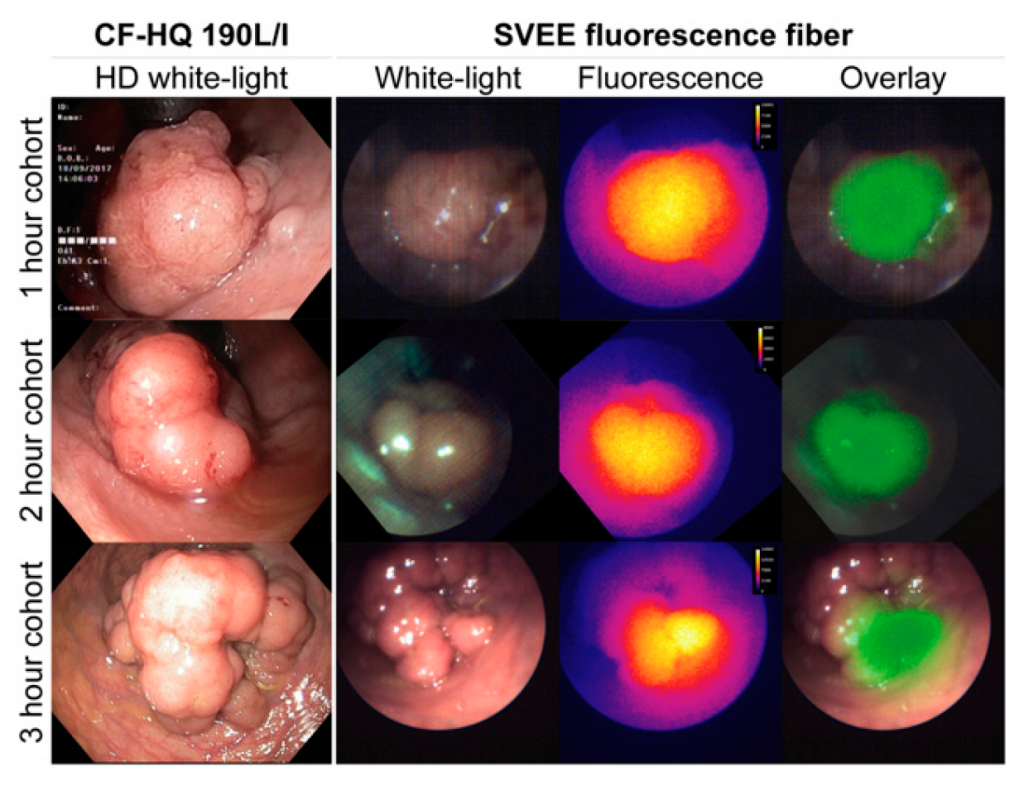
The work carried ou as part of Phase IIb stage of development of EMI-137 has showed FME using EMI-137 appeared to be safe and
feasible within a 1- to 3-h dose-to-imaging interval. No clinically
significant differences were observed among the cohorts, although
a 1-h dose-to-imaging interval was preferred from a clinical perspective.
Future studies will investigate EMI-137 for improved colorectal
polyp detection during screening colonoscopies.
For the full paper, follow the link: http://jnm.snmjournals.org/content/61/10/1435.abstract
